Standard enthalpy change of formation data table These tables include heat of formation data gathered from a variety of sources including the primary and secondary literature as well as. The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements.

For Many Reactions The Change In Enthalpy Equals Or Is Very Close To Change In E Cases 1 Reactio Teaching Chemistry Chemistry Education Chemistry Classroom
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements.

. Standard Enthalpies of Formation. Standard Enthalpy of Combustion. The standard condition in a thermodynamic reaction occurs when 1 mole of a substance is formed at the most stable state of 1 atm.
378 rows The standard enthalpy of formation ΔH0f of a compound is the change in enthalpy that accompanies the formation of 1 mole of a compound from its elements with all. The symbol of the standard enthalpy of formation is ΔH f. The standard enthalpy of formation for the.
It is the enthalpy change when a compound is formed from its constituent pure elements at standard. The standard enthalpy of formation of CO 2 g is 3935 kJmol. The standard enthalpy of formation at 25C 29815 K for 1 mol of the substance in its given state g gas and l liquide from its elements in their standard state.
There are two approaches to doing this. As mentioned on the previous page using Hess Law makes it possible to calculate many D Hs from just a few reactions for which D H is known. Standard enthalpy of formation or heat of formation ΔHof is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states.
The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions from its pure. Standard Enthalpy of Formation. ΔHfº is the standard enthalpy of formation for a compound.
The standard enthalpy of formation of a substance is the enthalpy change that occurs when 1 mole of the substance is formed from its constituent elements in their standard. The standard enthalpy of formation ΔH of is the enthalpy change for a formation equation when all substances are in their standard states. Pressure and 29815 K temperature.
227 rows ΔH f. Frequently Asked Questions FAQs. The standard enthalpies of formation of carbon dioxide and liquid water are 39351 and 28583 kJmol 1 respectively.
C s graphite O 2 g CO 2 g ΔH f ΔH 298 -393509. Having a library of values for standard enthalpies of formation allows calculating the enthalpy of reaction without doing calorimetric analysis. This is the enthalpy change for the exothermic reaction.
Standard enthalpy of formation is defined as the enthalpy change when one mole.

Standard Enthalpies Of Formation H At 298k Ap Chemistry Chemistry Fluoride

Heat Of Formation Delta H F The Heat Of Reaction When 1 Mol Of A Compound Is Produced From Its Elements Culture Generale Physique Chimie Chimie
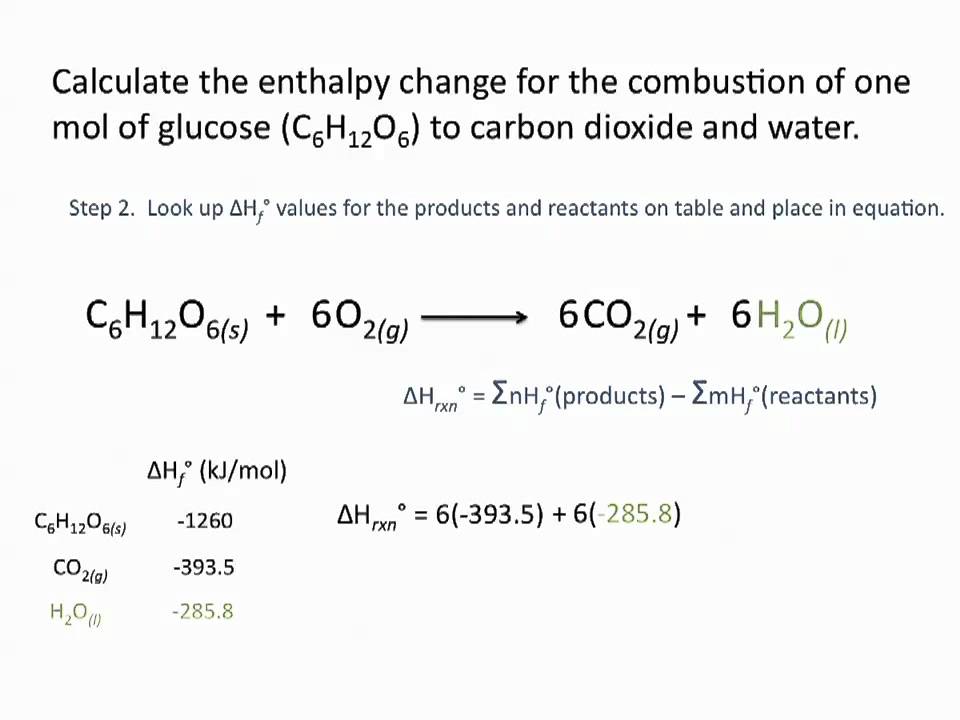
Enthalpies Of Formation Chemsitry Tutorial Science Chemistry Chemistry Tutorial

Finding Change In Enthalpy Of A Reaction From Change In Formation Values Of Reactants And Products By Appl Teaching Chemistry Chemistry Education Ap Chemistry
0 Comments