First the steric number of the central atom is. Also describe the electron pair geometry.

Physical Chemistry Bonding Flashcards Quizlet
To view the full text of the section click its citation.

. The basic geometry for a molecule containing a central atom with six pairs of electrons is octahedral. 388 chApTer 9 Molecular Geometry and Bonding Theories Count the number of electrons in a delocalized p systemSection 96 Explain the concept of bonding and antibonding molecular orbitals and draw examples of s and p MOs. As we replace bonding pairs with nonbonding pairs the molecular geometry changes to square pyramidalfive bonding and one nonbonding to square planar four bonding and two nonbonding.
There are three electron domains and this gives SO 2 an sp 2 hybridization. According to the VSEPR theory the Oxygen atoms are repelled by each other and the lone pair thus forming a bent molecular shape. A CF 4 - tetrahedral b BeBr 2 - linear c H 2 O - tetrahedral d NH 3 - tetrahedral e PF 3 - pyramidal.
Examples include PCl 5 and AsF 5. And the molecular polarity. This is what gives the molecules their shape.
As a result the molecular shape of IF5 is square pyramidal and PF5 is trigonal bipyramidal. As such the bond angle of SO 2 is 119. A NO 2-- trigonal planar b ClO 4-- tetrahedral.
4 Legislation makes new law to the society. ELECTRON PAIR MOLECULAR SHAPE AND POLARITY FOR AsH5 appeared first on Smashing Essays. Check the link it is a sheet describing the different types of electron and molecular geometry.
This is in contrast to the electronic geometry which describes the shape of all electron regions. A trigonal pyramidal D see-saw B tetrahedral E trigonal planar It is also known as Lewis dot structures which represent the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Which of the following best describes how the electron-pair geometry of a molecule is determined.
Look at the second N atom. Predict the molecular geometry and polarity of the SO2 molecule. In NH3 and H2O there are 1 and 2 lone pairs respectfully so more repulsion exists between the bond and lone pairs as.
Choose the species that is incorrectly matched with the electronic geometry about the central atom. Therefore the hybridization of Sulfur Dioxide is sp 2. Silane also known as monosilane is the simplest of all the chemical compounds belonging to silane groups which refer to binary silicon-hydrogen and organosilicon compounds having terminal hydrides.
In the geometry three atoms are in the same plane with bond angles of 120. Electron pair geometry and molecular geometry wont be the same if there are lone pairs involved. For each of the Lewis structures that follow a write the name of the electron group geometry around each atom that has two or more atoms attached to it b draw the geometric sketch of the molecule including bond angles and c.
Oxygen colorred3 This atom has two atoms directly attached and two lone pairs. A molecule of an element is composed of exactly two types of atoms. The type is AX3E0 so the electron -pair geometry is triangular planar and the approximate O CN angle is 120.
Its electron geometry and its molecular geometry are both tetrahedral as in methane. A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. WHAT IS THE LEWIS DOT STRUCTURE ELECTRON PAIR MOLECULAR SHAPE AND POLARITY FOR AsH5.
Molecular geometry or molecular shape is an important concept that we need to decipher while we are learning the chemical bonding of any chemical composition. How Do You Show Which Element. While Lewis Structure gives us an idea about the internal bond types and valence electron sharing inside a given molecule it can only explain a two-dimensional.
The theory says that repulsion among the pairs of electrons on a central atom whether bonding or non-bonding electron pairs will control the geometry of the molecule. The Lewis structure for CS2 is. Molecular shape is only discussed when there are three or more atoms connected diatomic shape is obvious.
This molecule is similar to CH4 in that it has an overall tetrahedral shape. SiH4 is the structural composition of silanesilicane. An example of this geometry is SF 6.
This document is a tool that explains what each code. Which is the best description of a molecule. Its electron geometry and its molecular geometry are both trigonal planar.
Using VSEPR theory predict the molecule shape of a molecule that contains 2 electron groups. NF3 Molecular Geometry. Third the electron-pair geometry is determined by assigning the steric number to the central atom.
Section 97 Draw molecular orbital energy-level diagrams and place electrons into them to obtain the bond orders and electron configurations. First a Lewis dot structure is created. Some elements in Group 15 of the periodic table form compounds of the type AX 5.
SO2 Molecular Geometry. CH4 has no lone pairs of Electrons on the central atom so the optimal molecular shape would be tetrahedral with bond angels of 1095. What best describes the electronic geometry of the molecule.
In IF5 there is a lone pair of electrons on iodine where as in a PF5 there are no lone pairs on phosphorus. Define Electronegativity And Describe Its Periodic Trends. Choose the molecule that is incorrectly matched with the electronic geometry about the central atom.
Look at the C atom. Annotated codes are very useful because they describe a statutes history list and describe cases that interpret a statute provide citations to relevant regulations and. Carbon colorred2 This atom has three atoms directly attached and no lone pairs.
AJR Ch10 Molecular Geometrydocx Slide 1 Chapter 10 Molecular Geometry Ch9 Jespersen Ch10 Chang The arrangement of the atoms of a molecule in space is the molecular geometry. There are two C-S double bonds at the CS2 molecular geometry. SiH4 Lewis Structure Molecular Geometry Hybridization and Polarity.
It helped me a lot. What best describes the molecular geometry of the molecule. A molecule of a compound is composed of at least two types of atoms.
N N O N N O 180. A molecule of a compound is composed of only one type of atom. Electron Geometry Hybridization Molecular Geometry VSEPR class Approximate Bond Angles 5 0 Trigonal Bipyramidal AX 5 4 1 Seesaw AX 4 E 3 2 T-Shaped AX 3 E 2 2 3 Linear AX 2 E 3 180 6 0 Octahedral AX 6 5 1 Square Pyrimidal AX 5.
Second the electron pairs are arranged to minimize repulsion. A molecule of an element is composed of at least two types of atoms. Exercise 123 - Molecular Geometry.
The other two atoms are on opposite ends of the molecule. The molecular geometry around the central atom. The valence shell electron-pair repulsion theory abbreviated VSEPR is commonly used to predict molecular geometry.
The type is AX3E1 so the electron -pair geometry is tetrahedral and the a pproximate HNH angle is 1095.

So32 Molecular Geometry Shape And Bond Angles Molecular Geometry Chemistry Help Molecular

Physical Chemistry Bonding Flashcards Quizlet

Co32 Molecular Geometry Shape And Bond Angles Carbonate Ion Molecular Geometry Molecular Geometry

Physical Chemistry Bonding Flashcards Quizlet

Solved Consider The Following Molecule Sebr What Best Chegg Com
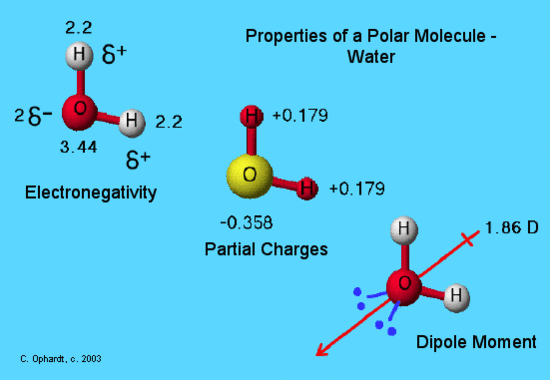
3 7 Geometry And Dipole Moment Chemistry Libretexts

Bond Angles Are The Angles Between Adjacent Lines Representing Bonds And They Greatly Contribute To A Molecule S Mole Chimie Genie Mecanique Science
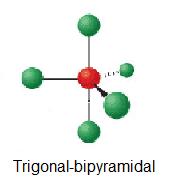
3 7 Geometry And Dipole Moment Chemistry Libretexts

So4 2 Lewis Structure How To Draw The Lewis Structure For So4 2 Sulfate Ion This Step By Step Exp Chemistry Classroom Teaching Chemistry Chemistry Lessons
0 Comments